FDA Allows PM to Market IQOS as 'Reduced Risk' - Tobacco Asia

Are High-Tech Tobacco Products Healthier? FDA Wants More Data - Bloomberg

Heated Tobacco Products - TobaccoTactics

PMI's IQOS and cigarette ads in Israeli media: a content analysis across regulatory periods and target population subgroups
U.S. FDA's tobacco stance faces test with Philip Morris iQOS device

Philip Morris Allowed to Say IQOS Reduces Harmful Exposure - Bloomberg

The FDA authorizes the sale of IQOS 3 in the U.S.
Philip Morris's Cigarette Alternative Could Hit U.S. in 2017 - Bloomberg

PDF) Heated tobacco product regulation under US law and the FCTC
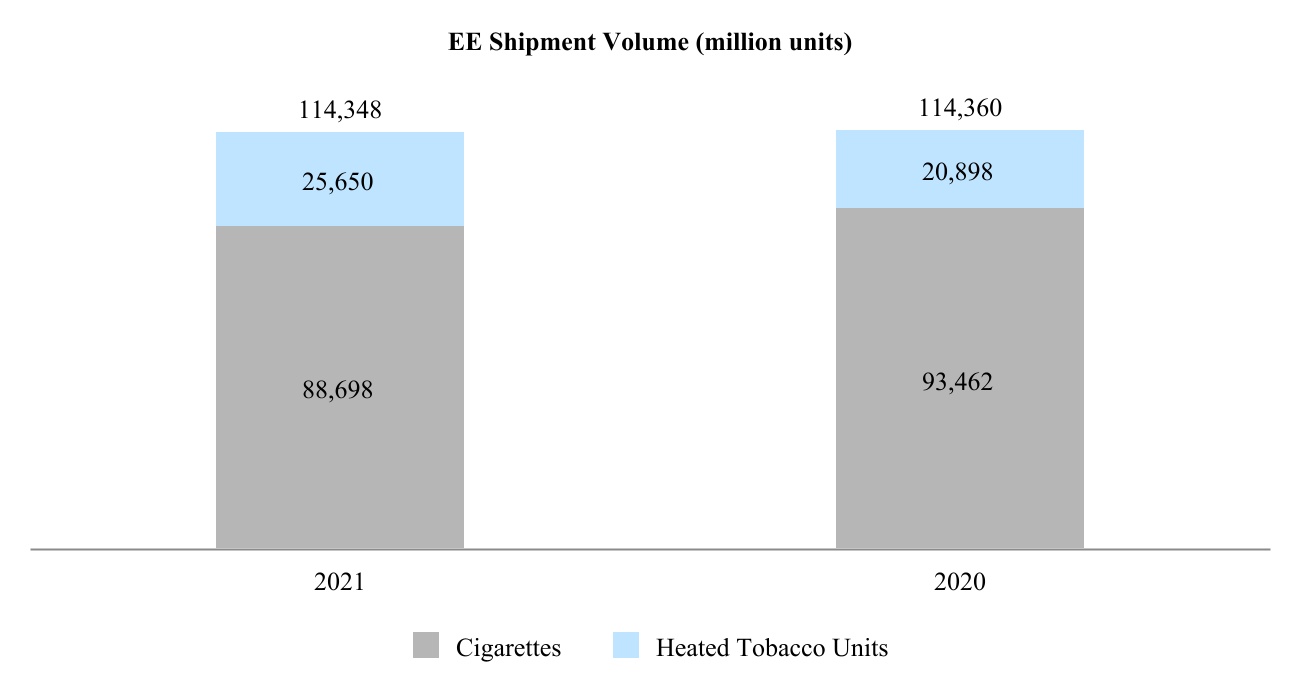
pm-20211231

FDA clears Philip Morris' iQOS, Altria prepares to sell the heated tobacco device in the US

Big Tobacco Has a Lot Riding on FDA's Stance Toward IQOS, the Latest Cigarette Alternative - WSJ
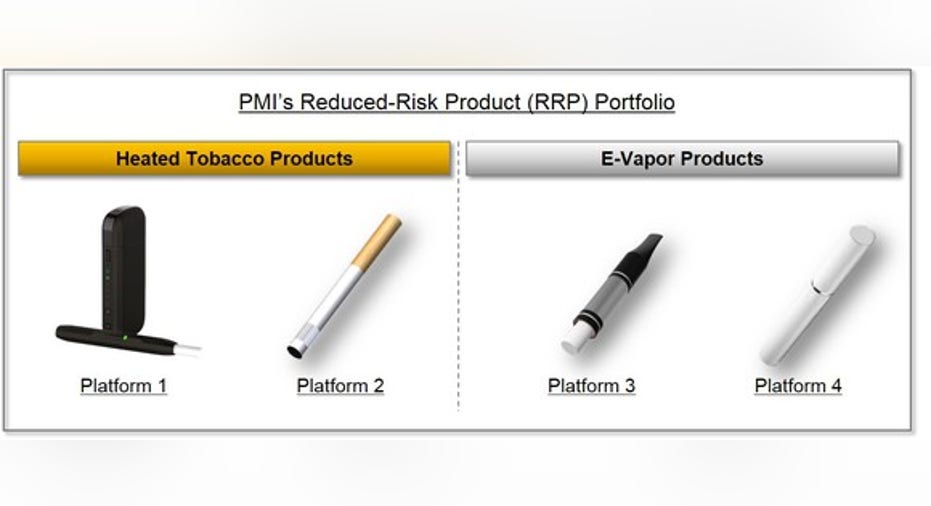
What Philip Morris International's FDA Application Means for iQOS

Cigarette company Philip Morris tries to sell idea of 'safer' tobacco









