
Solved overall reaction: 2NO(g)+O2(g)→2NO2(g) Step

Consider this reaction : 2NO(2)(g)+O(3)(g)rarrN(2)O(5)(g)+O(2)(g)

For a reaction, 2NO(g) + O2(g)→ 2NO2(g) Rate = k [ NO ]^2 [ O2 ] if the volume of the reaction vessel is doubled, then the rate of the reaction
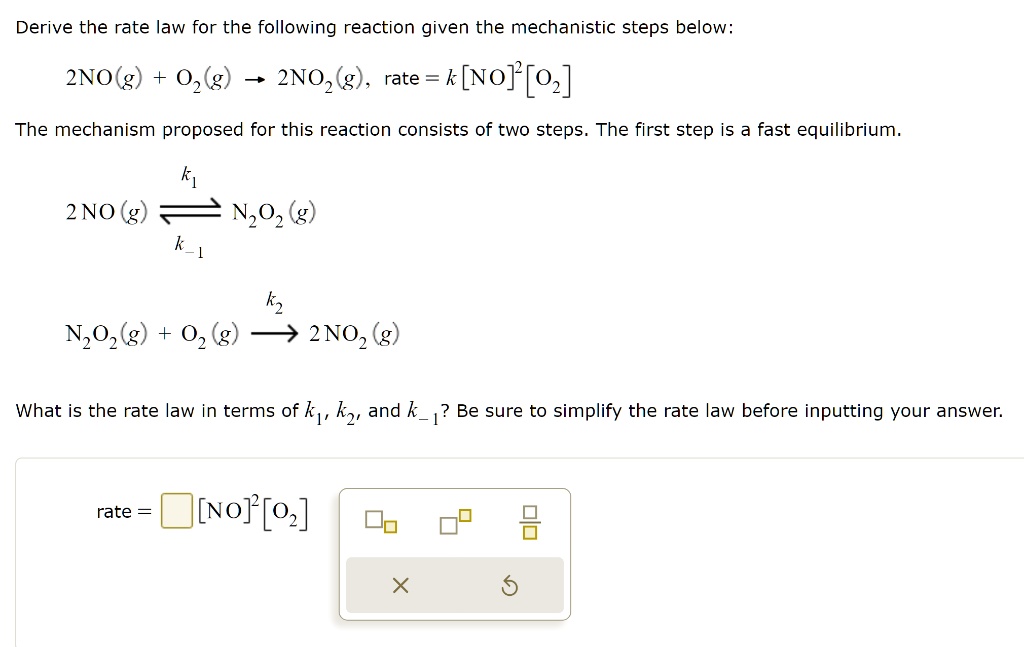
SOLVED: Derive the rate law for the following reaction given the mechanistic steps below: 2NO(g) + O2(g) = 2NO2(g), rate = k[NO][O2] The mechanism proposed for this reaction consists of two steps.

The addition of NO accelerates the decomposition of N2O, possibly

Solved 4) For the overall reaction, 2NO(g) + O2(g) →
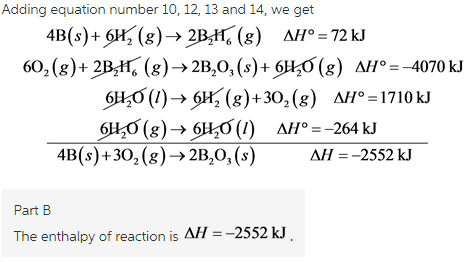
Calculate the enthalpy of the reaction 2NO(g)+O2(g)→2NO2(g) - Home Work Help - Learn CBSE Forum
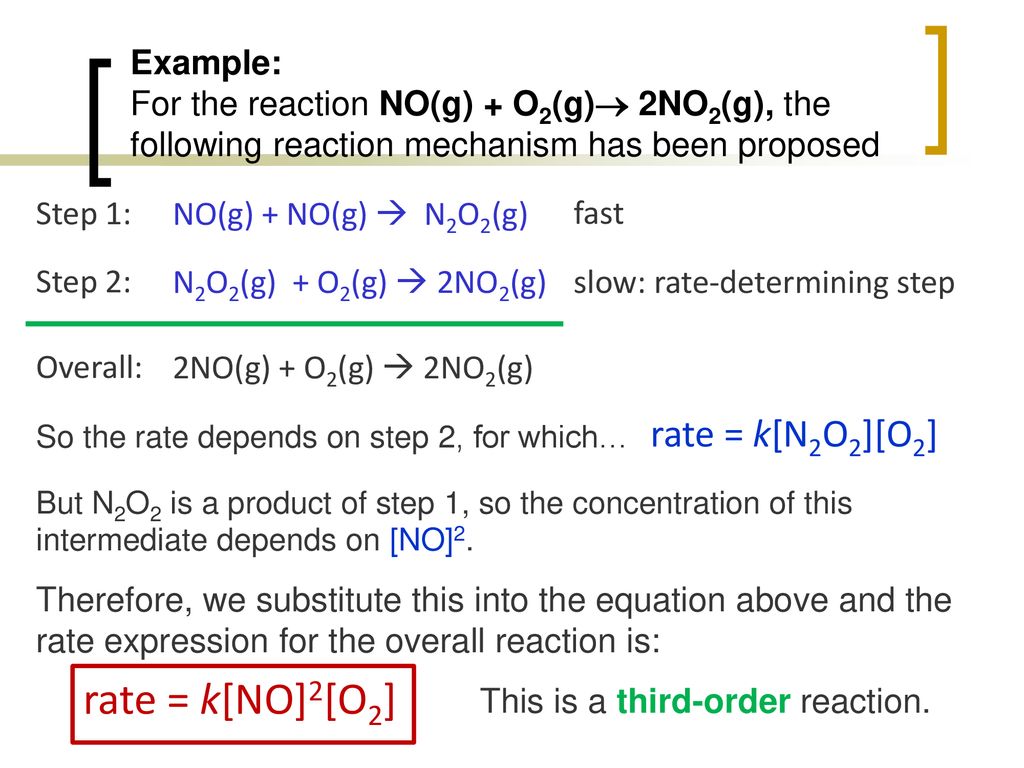
Part 3: Reaction Mechanisms - ppt download

Reaction Mechanisms The sequence of events that describes the actual process by which reactants become products is called the reaction mechanism. Reactions. - ppt download
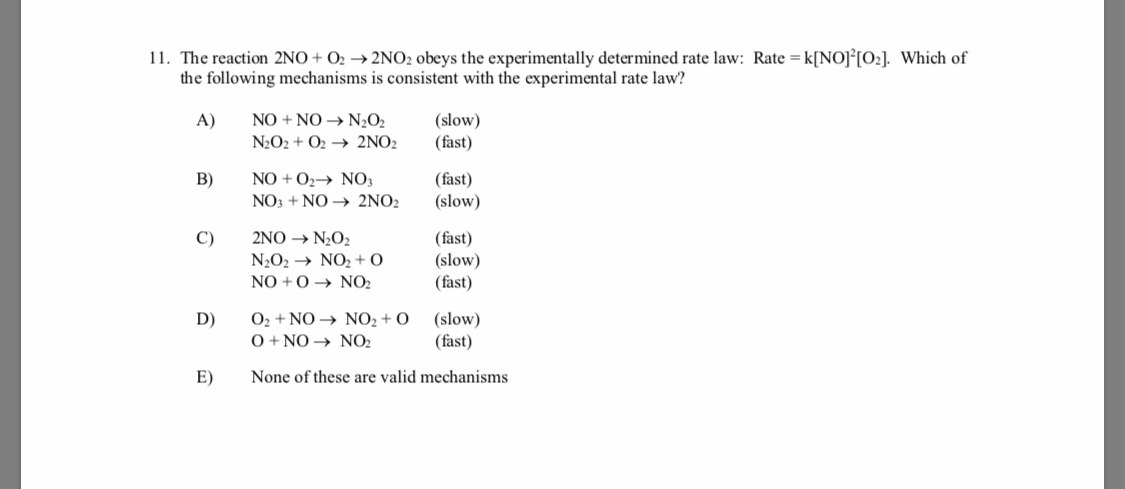
Answered: 11. The reaction 2NO + O2 → 2NO2 obeys…

Answered: 4.) The overall reaction 2NO2(g) +…
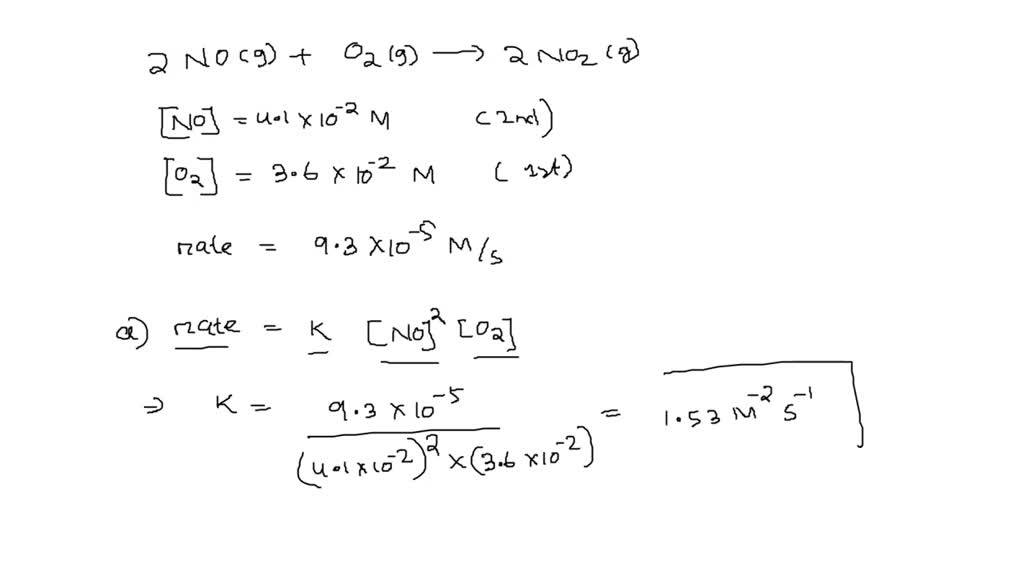
SOLVED: The reaction 2NO(g) + O2(g) â†' 2NO2(g) is second order in NO and first order in O2. When [NO] = 4.1×10^-2 M and [O2] = 3.6×10^-2 M, the observed rate of

For the reaction mechanism given in Problem 18-12, classify









