
FDA Repeatedly Rejected Safety Claims About Philips Breathing Machines, Emails Show — ProPublica
As Philips reassured patients that millions of recalled machines were safe, internal emails show federal regulators privately told the company its testing didn’t account for the impact of long-term harm from tainted devices.

Philips CPAP Recall Support Group, Weekend update

Millions of people used tainted breathing machines. The FDA failed to use its power to protect them.

FDA repeatedly rejected safety claims made after recall of a CPAP but waited to alert the public, emails show - Government Executive
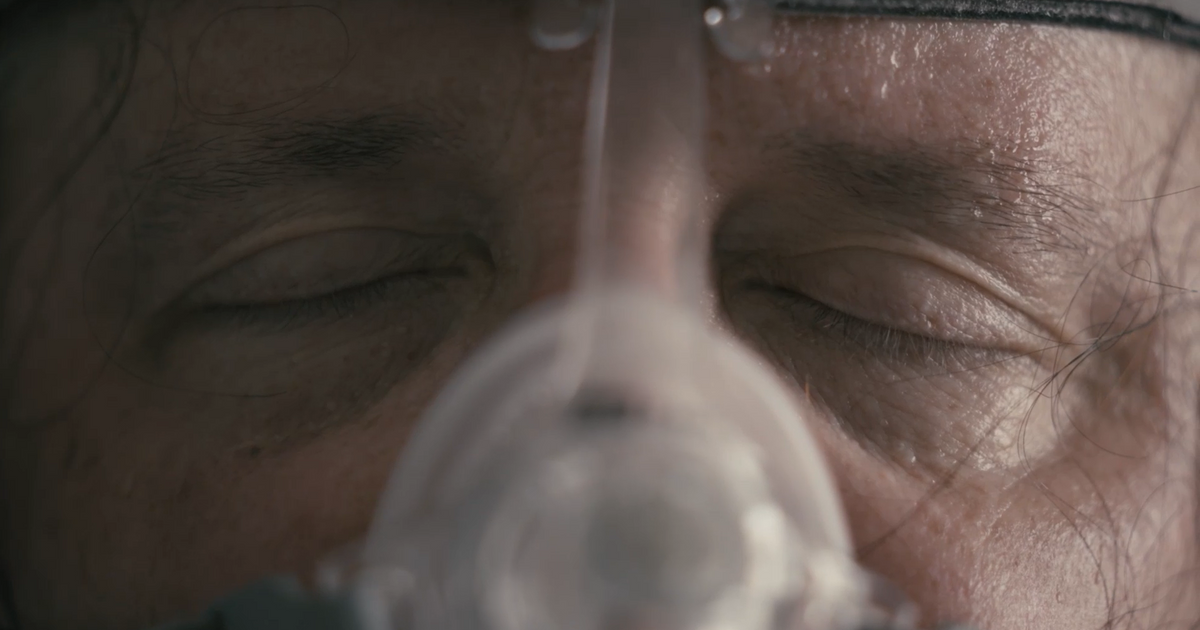
Philips Kept Complaints About Dangerous CPAP Machines Secret While Company Profits Soared — ProPublica
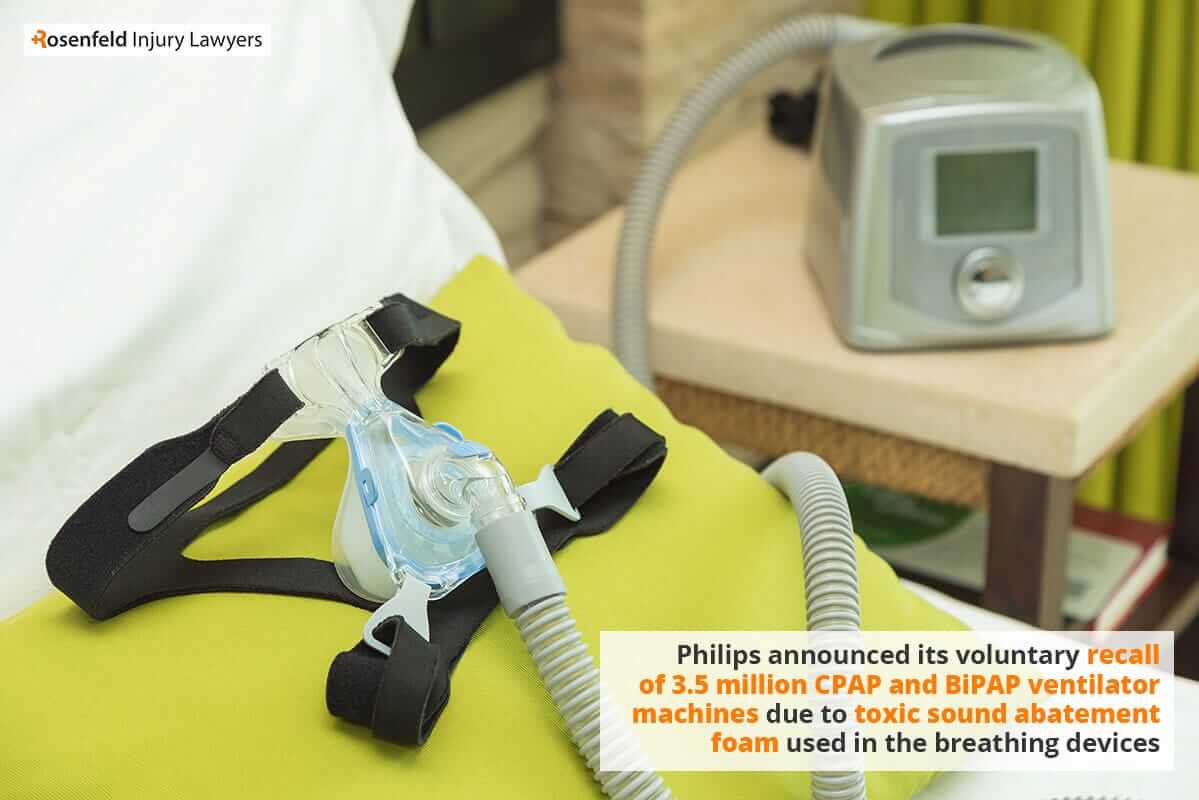
Philips CPAP Lawsuit Lung Damage & Cancer Claims 2023

Philips CPAP Recall Support Group
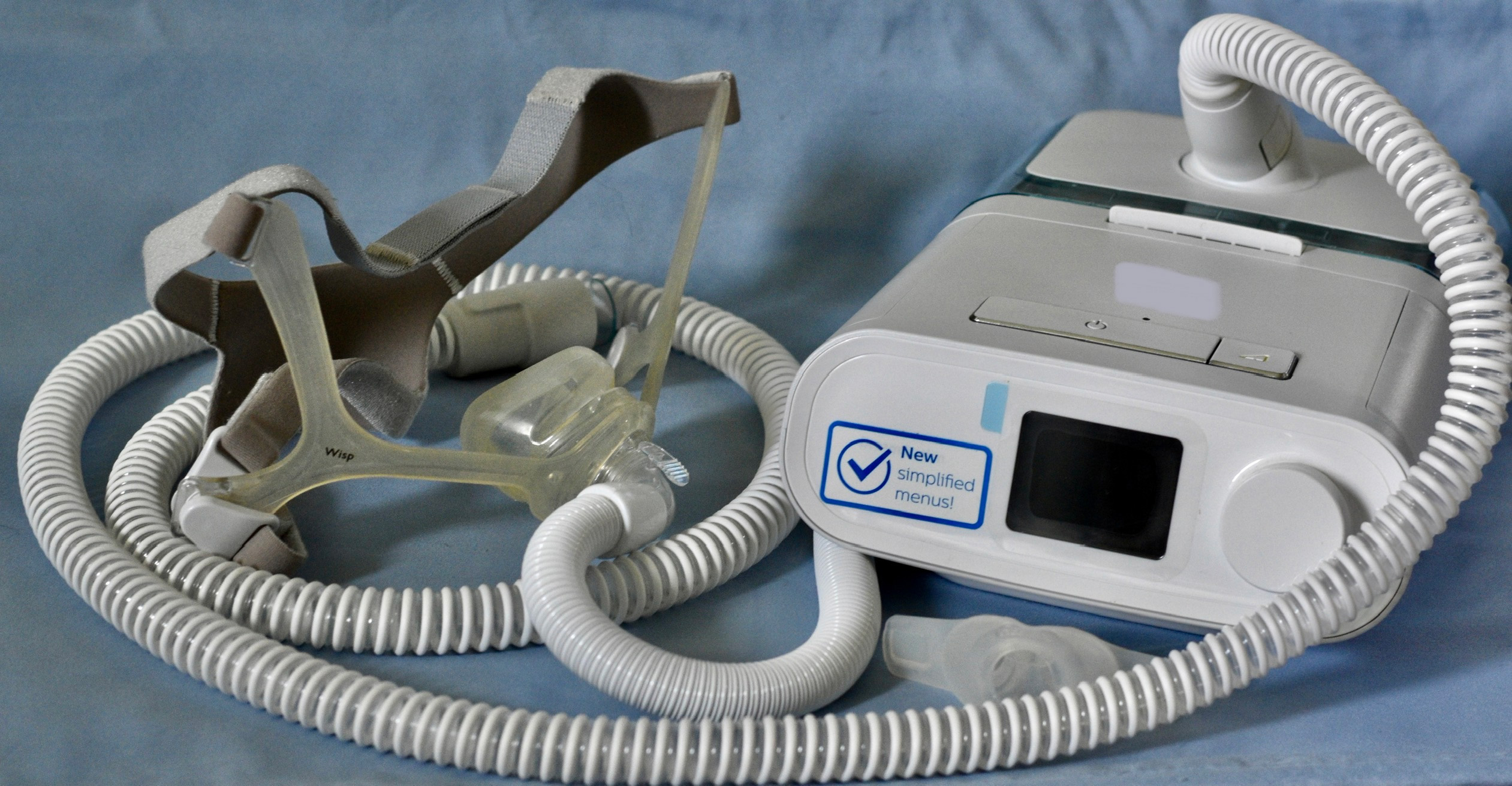
Philips Ignored Complaints About Breathing Machines Despite Evidence of Hazard

FDA Orders Philips Respironics to Notify Consumers of CPAP Recall - Leaders in Mass Torts

Brian Pierce MD (@brianpierce@) - Med-Mastodon

ProPublica on LinkedIn: How Verified Accounts on X Thrive While Spreading Misinformation About the…









